Performance of devices in medical applications (in vitro)
 We determine the following performance parameters e.g. of hemodialyzers:
We determine the following performance parameters e.g. of hemodialyzers:
- Clearance of low molecular weight solutes in aqueous solutions according to ISO 8637
- Clearance of low molecular weight plasma proteins (LMWP) from blood and plasma
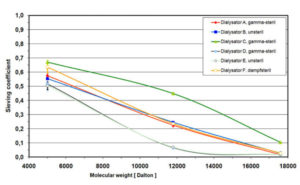
- Determination of sieving coefficients of plasma proteins from plasma or human blood according to ISO 8637
- Albumin loss into dialysate during hemodialysis und hemodiafiltration (online-HDF)
- Determination of transmembrane pressure (TMP) in aqueous solution, plasma and human blood.
- Determination of ultrafiltration rate (UFR) and ultrafiltration coefficient (KUF) in aqueous solution, plasma and human blood.
LMWP clearances or albumin loss is determined using freshly donated human blood or plasma supplemented with concentrated hemofiltrate obtained from end-stage renal disease (ESRD) patients. By this approach donor blood gets closer to the properties of uremic blood (e.g. with regard to the concentration of uremic toxins like β2-microglobulin).